-
Suprachoroidal cavity drug delivery technology: an easy-to-operate
and effective posterior segment drug delivery mode
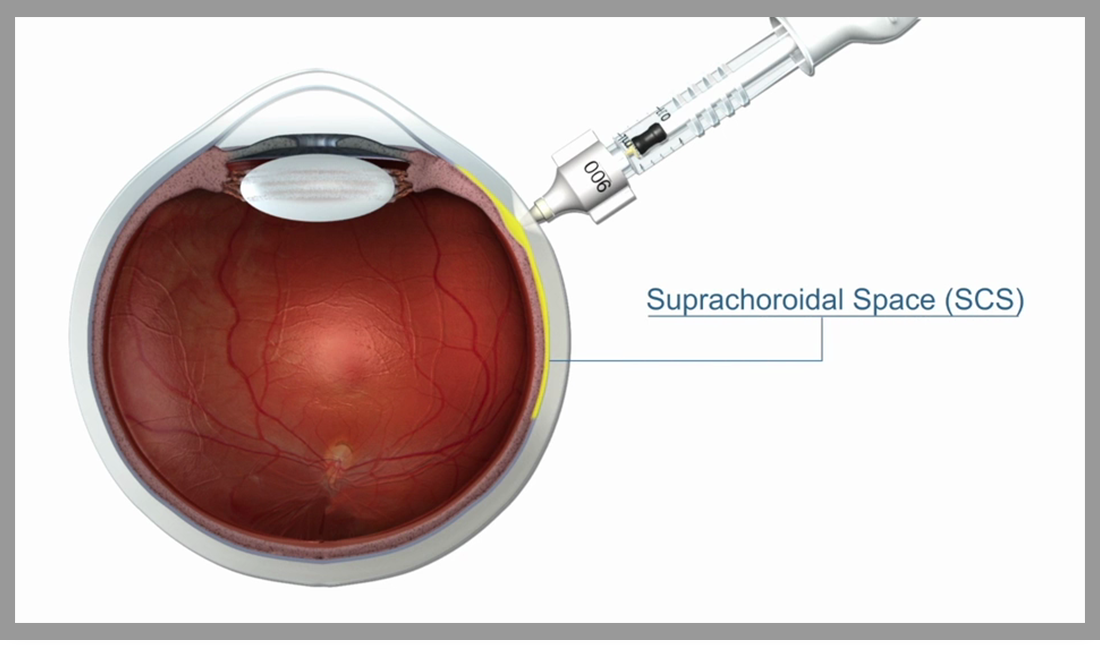
Since a variety of eye diseases that seriously harm visual function involve the retina and choroid, drug delivery in the posterior segment of the eye has always been an important research topic for ophthalmologists and drug delivery scientists. However, due to the particularity and complexity of the anatomy and pharmacokinetics of the eyeball, the challenge of drug delivery in the posterior segment of the eye continues. There are systemic and multiple local administration methods in current ophthalmology clinical practice, including eye drops, perisphere or intravitreal injection, etc. Each method has its own unique advantages and risks. Suprachoroidal cavity injection is a new type of ocular administration method that directly delivers the drug to the suprachoroidal cavity and diffuses it to the macula, retina, choroid and other frequent sites of serious eye diseases. XIPERE™ (Triamcinolone Acetonide Suprachoroidal Injection Suspension, developed and commercialized in China by XM Bio) is a patented suspension of corticosteroid triamcinolone acetonide, through the unique SCS Microinjector™ (Suprachoroidal Microinjector™) ) Used to administer to the posterior segment of the eye to treat uveitis macular edema. Injection of the suprachoroidal space can quickly and fully disperse the drug to the back of the eye, which is expected to make the drug's effect more durable and minimize the adverse effects on normal tissues in the eye. Today I bring you two new articles on suprachoroidal administration published at the 2020 ARVO Annual Meeting. Let us explore this new mode of administration in the posterior segment of the eye.
Injecting TA into the suprachoroidal space to treat non-infectious uveitis

Purpose: The treatment of uveitis often requires local combined systemic drug therapy. This study will explore the efficacy of XIPERE™ (a patented suspension of triamcinolone acetonide) in the suprachoroidal space for patients with non-infectious uveitis who have or have not received other systemic treatments.
Methods: 160 patients were randomly assigned to the XIPERE™ suprachoroidal injection group or the sham treatment group in a 3:2 ratio, and received 2 doses at baseline and 12 weeks respectively. Patients who are receiving low-dose corticosteroids or stable doses of immunomodulatory therapy are allowed to be included in this study. The primary study endpoint was the percentage of patients with EDTRS best corrected visual acuity (BCVA) increased by 15 letters or more. Secondary endpoints included the average change from baseline in BCVA and central subregional macular thickness (CST). Post-hoc analysis was performed on patients who received systemic corticosteroid and/or non-steroid therapy at baseline and patients who did not receive systemic therapy to evaluate the improvement of BCVA and CST.
Results: Overall, 46.9% of the patients in the treatment group achieved visual improvement of more than 15 letters at the 24th week, while the proportion of the control group was only 15.6%. Compared with baseline, BCVA improved by 13.8 letters in the treatment group and 3.0 letters in the control group. In addition, the mean CST of the treatment group was reduced by 152.6 μ m, while that of the control group was only 17.9 μ M. In the post-mortem analysis, 68 patients in the treatment group and 49 patients in the control group did not receive additional systemic treatment (systemic corticosteroids or non steroids). In these patients, BCVA improvement was 15.6 and 4.9 letters in treatment group and control group at 24 weeks (P < 0.001), and CST reduction was 169.8 μ m and 10.3 μ m (P < 0.001), respectively. In addition, 28 patients in the treatment group and 15 patients in the control group were receiving systemic corticosteroids and / or non steroids at baseline. Among these patients with baseline treatment, BCVA increased by 9.4 letters in the treatment group and decreased by 3.2 letters in the control group at week 24 (P = 0.002), and the decrease of CST was 108.3 μ m and 43.5 μ m in the treatment group and the control group respectively (P < 0.001).
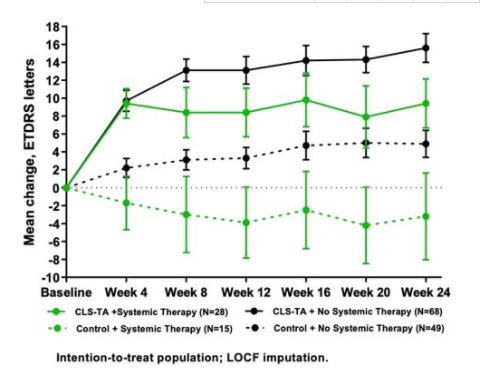
Conclusion: the results of post event analysis also confirmed the results of the pre-set study. Compared with the control sham treatment, xipere ™ It provides clinically significant improvements in BCVA and CST, regardless of whether the patient has received systemic immunosuppressive therapy.
2. Operating force of the SCS microinjector in the suprachoroidal space

Objective: suprachoroidal injection with SCS microinjector is a new technique to deliver drugs to the posterior segment of the eye, and tactile feedback with loss of resistance is the key to success. The resistance caused by any operation of the syringe will make it difficult for the user to distinguish whether the resistance is caused by the anatomical part of the needle or from the internal friction force of the syringe. The purpose of this study was to quantify the thrust required to operate SCS microsyringes under controlled laboratory conditions and to compare with the recommended force required to operate a standard syringe.
Methods: a SCS microinjector with a 1100 μ m long needle (30g) was used to transfer the injection (air, distilled water and xipere) into the syringe Gamma -40 mg / ml triamcinolone acetonide suspension) was filled to the nominal volume (0.1 ml) and then connected to a dynamometer to measure the thrust required to discharge the injection from the syringe at a controlled rate. The injection was completed in 5-10 seconds according to the instructions of the syringe, and the sliding force was calculated respectively (the force required to make the continuous plunger move inside the syringe) and the separating force (the force required to start the plunger moving in the syringe). The measurement results were compared with the standard reference values in ISO 7886-1:2017 "sterile hypodermic syringes for single use".
Results: SCS microinjector was used to inject air, water and xipere Gamma -The sliding forces required by TA were 0.19n, 0.23n and 0.73n respectively (P < 0.001). The injection sliding force of the three injections were significantly lower than the reference maximum value of 5N in ISO 7886, indicating that the start-up of SCS microinjector is easier than that of standard syringe. The average separation force of injection air and water was 0.43n and 0.41n (P = 0.314), respectively. However, no significant separation force was detected in xipere TA injection. Similar to the sliding force, the separation force of SCS microinjector is much lower than the standard reference value of 10N.
Conclusion: the thrust force of SCS microinjector is far less than that of low volume hypodermic syringe recommended by international standards. This may minimize the intrinsic resistance of the syringe to improve the usability of the SCS microinjector, so that the injector can obtain more accurate tactile feedback when reaching the suprachoroidal cavity.
To sum up
From the above two documents, we can conclude that the SCS microsyringe suprachoroidal injection is an easy-to-operate and effective technique for drug delivery to the posterior segment of the eye. Injecting XIPERE™-TA through the suprachoroidal space has a good therapeutic effect on non-infectious uveitis with different baseline treatment conditions.
(At the end of the article, you can insert the product license-in news link of our official Weibo)
References
1. Christopher R Henry, Thomas Ciulla, Colette Hall; Results from the Phase 3 PEACHTREE Clinical Trial: Systemic Therapy and the Efficacy of CLS-TA, a Post-Hoc Analysis. Invest. Ophthalmol. Vis. Sci. 2020;61(7):5367.
2.Nathan Fisher, Cherry Wan; Suprachoroidal Delivery with the SCS Microinjector™: Characterization of Operational Forces. Invest. Ophthalmol. Vis. Sci. 2020;61(7):24.
For more related reading, please visit https://www.clearsidebio.com/publications.htm
To report a potential adverse event, please refer to the contact information below:
Global: globalsafety@arcticvision.com
Mainland China: safety.cn@arcticvision.com
Hong Kong, China: safety.hk@arcticvision.com